CHEM21 Case Study: Synthesis of 2-fluoromalonate Esters Via Selective Direct Fluorination
This case study was provided by Prof. Graham Sandford from the Centre of Sustainable Processes at Durham University.
Although many pharmaceutically relevant molecules contain fluoro- or trifluoromethyl-aromatic functionalities, drug development now has evolved to require chemical entities that contain fluorine functionality at unaccessible sites and thus there continues to be a demand for the development of efficient, selective and economically viable methods for fluorination on industrial scale.[1][2][3][4]
Large scale manufacture of fluorinated compounds are carried out using expensive anhydrous hydrogen fluoride (aHF). However, the highly corrosive nature of this reagent limits fluorination reactions to structurally simple organic substrates, which have to be pre-functionalised with nitro- or chloro- groups through multistep procedures.[5] Ideally, the most efficient and direct way of achieving fluorination on large scale would be the selective conversion of a carbon-hydrogen bond to a carbon-fluorine bond using inexpensive fluorine gas.[6] Despite the recent advances seen in selective fluorination methods for both batch and flow processes, the use of fluorine gas for life science product manufacture has thus far been limited to the production of 5-fluoracil[7] as well an intermediate in the synthesis of Voricanazole (V‑Fend, Pfizer).[8]
2-Fluoromalonate esters represent a class of potentially versatile building blocks for the synthesis of fluorinated compounds; various alkylations,[9] Michael additions,[10][11][12] and heterocycle formation reactions[13][14] have been reported for them, which gives a good indication of their utility in organic synthesis. There are three reasonable, low-cost synthetic strategies available for large scale manufacture of diethyl 2-fluoromalonate; the reaction of ethanol with hexafluoropropene (HFP),[15] halogen exchange (Halex)[16] and a selective direct fluorination process[17] (Scheme 1).
CHEM21 researchers have assessed and optimised the direct fluorination of diethyl malonate, catalysed by copper nitrate in flow, with the goal of intensifying the transformation and reducing its environmental impact. The optimised system for the selective fluorination process is shown in Scheme 2 and was applied successfully to related malonate esters in excellent yields.[13]
![Scheme 1: Three reasonable, low-cost synthetic strategies available for large scale manufacture of diethyl 2-fluoromalonate [5] Reproduced under license: Creative Commons Attribution 3.0](https://learning.acsgcipr.org/wp-content/uploads/2022/07/c86dcf788c4e314e2b74e17dd8659ba0.jpg)
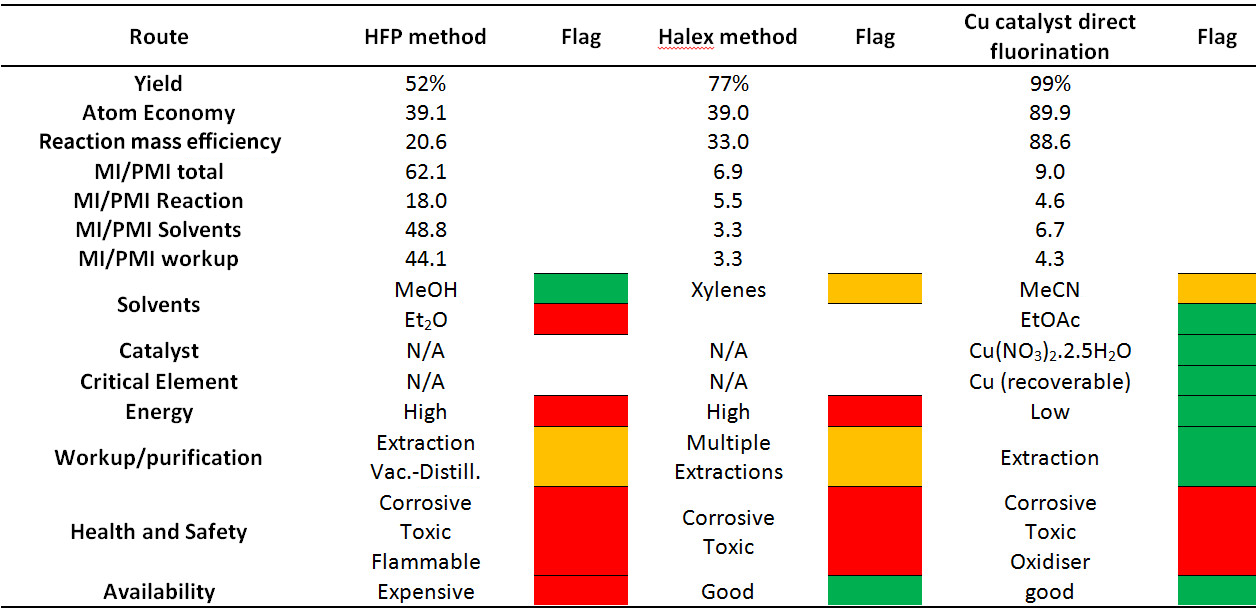
The CHEM21 researchers went further and investigated the green metrics of the optimised direct fluorination process as well as the other two approaches shown in Scheme 1. The group applied the CHEM21 metrics toolkit [18] at first pass to all three approaches, the results for which are shown in Table 1.
![Scheme 2: Optimised copper nitrate catalysed direct fluorination of diethyl malonate [5] Reproduced under license: Creative Commons Attribution 3.0](https://learning.acsgcipr.org/wp-content/uploads/2022/07/fabeb6480fa8fa654c84dd640608175d.jpg)
The resultant metrics for the direct fluorination approach shows low material intensity with a PMI value of below 10, and other green metrics for this approach compare favourably with those of the HFP and Halex method in terms of environmental impact. These results demonstrate that the new optimised direct fluorination approach serves as an excellent benchmark figure for an efficient, effective and environmentally benign approach to the synthesis of fluoromalonates.
- B. Baasner, H. Hagemann and J. C. Tatlow, Houben-Weyl Organofluorine Compounds, 2000, E10a.
- R. D. Chambers, Fluorine in Organic Chemistry, Wiley-Blackwell, Oxford, 2004.
- K. Uneyama, Organofluorine Chemistry, Blackwell, Oxford, 2006.
- T. Liang, C. N. Neumann and T. Ritter, Introduction of Fluorine and Fluorine-Containing Functional Groups, Angew. Chem. Int. Ed., 2013, 52, 8214-8264.
- A. Harsanyi and G. Sandford, Fluorine gas for life science syntheses: green metrics to assess selective direct fluorination for the synthesis of 2-fluoromalonate esters, Green Chem., 2015, 17, 3000–3009.
- G. Sandford, Elemental fluorine in organic chemistry (1997–2006), J. Fluorine Chem., 2007, 128, 90-104.
- Patent 4631342, 1986, 4631342.
- M. Butters, J. Ebbs, S. P. Green, J. MacRae, M. C. Morland, C. W. Murtiashaw and A. J. Pettman, Process Development of Voriconazole: A Novel Broad-Spectrum Triazole Antifungal Agent, Organic Process Research & Development, 2001, 5, 28-36.
- J. Dubois, C. Fourès, S. Bory, S. Falcou, M. Gaudry and A. Marquet, Synthesis of 5,5′-dihydroxyleucine and 4-fluoro 5,5′-dihydroxyleucine, the reduction products of 4-carboxyglutamic and 4-carboxy-4-fluoroglutamic acids, Tetrahedron, 1991, 47, 1001-1012.
- H. Li, L. Zu, H. Xie and W. Wang, Organocatalytic Enantioselective Conjugate Addition of 2-Fluoromalonate to Nitroolefins: Direct Synthesis of Chiral Fluorinated gamma-Nitro Carboxylic Acid Derivatives, Synthesis-Stuttgart, 2009, 1525-1530.
- B. Kyung Kwon, S. Mi Kim and D. Young Kim, Highly enantioselective conjugate addition of fluoromalonates to nitroalkenes using bifunctional organocatalysts, J. Fluorine Chem., 2009, 130, 759-761.
- X. Companyó, M. Hejnová, M. Kamlar, J. Vesely, A. Moyano and R. Rios, Highly enantioselective fluoromalonate addition to α,β-unsaturated aldehydes, Tetrahedron Lett., 2009, 50, 5021-5024.
- A. Harsanyi, G. Sandford and J. A. Yufit Dmitri S and Howard, Syntheses of fluorooxindole and 2-fluoro-2-arylacetic acid derivatives from diethyl 2-fluoromalonate ester, Beilstein J. Org. Chem., 2014, 10, 1213–1219.
- C. A. Fisher, A. Harsanyi, D. S. Sandford Graham and Yufit and J. A. K. Howard, Fluorotetrahydroquinolines from diethyl 2-fluoromalonate ester, Chimia, 2014, 68, 425–429.
- N. Ishikawa, A. Takaoka and K. M. Ibrahim, Preparation of 2-fluoromalonic esters and related compounds from hexafluoropropene, J. Fluorine Chem., 1984, 25, 203-212.
- Patent WO 2005019154, 2005, WO 2005019154.
- R. D. Chambers, M. A. Fox, D. Holling, T. Nakano, T. Okazoe and G. Sandford, Versatile Gas/Liquid Microreactors for Industry, Chemical Engineering & Technology, 2005, 28, 344-352.
- R. C. McElroy, A. Constantinou, L. C. Jones, L. Summerton and J. H. Clark, Towards a holistic approach to metrics for the 21st century pharmaceutical industry, Green Chem., 2015, 17, 3111-3121.