CHEM21 Case Study: Multicomponent Synthesis of Isothioureas
Reproduced Content
This case study was provided by Prof. Bert Maes’ ORSY team at the University of Stuttgart.
With important medicinal properties, isothioureas are an important class of compounds to the pharmaceutical industry, which have found use as anti-histamines, anti-bacterials, for treatment of peptic ulcers, HIV and influenza.[1][2][3][4][5] They are also crucial intermediates in the synthesis of guanidines [6] [7] (used in treatment of Lambert‑Eaton syndrome), with many routes to their synthesis reporting the formation of isothioureas as intermediates.[6] [7] Beyond pharmaceutical applications, isothioureas also have applications as agrochemicals as well as within the fine chemicals industry.
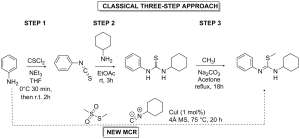
Classically S-alkyl isothioureas are prepared from the corresponding thioureas by S-alkylation. The most common intermediates in the synthesis of guanidines, S-methyl isothioureas are formed by reaction of the corresponding thioureas with the highly carcinogenic and neurotoxic methyl iodide (MeI).[8] The use of alkyl halides to furnish S-alkyl isothioureas presents a variety of disadvantages including the associated health risks, flammability and reactivity of the reagents.
Given the industrial significance of isothioureas and relative lack of research into greener approaches to their synthesis, the CHEM21 researchers have developed a multicomponent reaction for the synthesis of isothioureas from isocyanides, thiosulfonates and amines,[8] the classical approach requires three synthetic steps with associated chromatographic purification to achieve the alkylated isothiourea product, which the novel MCR approach can achieve in one step. It also circumvents the use of an alkyl halide for the S-alkylation (Scheme 1).
The novel methodology allows access to a variety of novel isothioureas, including S-aryl isothioureas which are difficult to achieve by other methods.[8] The method allows for the use of readily available isocyanides, thiosulfonates and (hetero)aromatic amines to achieve the target molecules in a single synthetic step. The methodology operates using a copper based catalyst, does not require air exclusion and operates under mild reaction temperatures.[8]
- A. Nicholson, J. D. Perry, A. L. James, S. P. Stanforth, S. Carnell, K. Wilkinson, C. M. Anjam Khan, A. De Soyza and K. F. Gould, In vitro activity of S-(3,4-dichlorobenzyl)isothiourea hydrochloride and novel structurally related compounds against multidrug-resistant bacteria, including Pseudomonas aeruginosa and Burkholderia cepacia complex, Int. J. Antimicrob. Ag., 2012, 39, 27-32.
- S. Harusawa, K. Sawada, T. Magata, H. Yoneyama, L. Araki, Y. Usami, K. Hatano, K. Yamamoto, D. Yamamoto and A. Yamatodani, Synthesis and evaluation of N-alkyl-S-[3-(piperidin-1-yl)propyl]isothioureas: High affinity and human/rat species-selective histamine H3 receptor antagonists, Bioorg. Med. Chem. Lett., 2013, 23, 6415-6420.
- E. P. Istyastono, S. Nijmeijer, H. D. Lim, A. van de Stolpe, L. Roumen, A. J. Kooistra, H. F. Vischer, I. J. P. de Esch, R. Leurs and C. de Graaf, Molecular Determinants of Ligand Binding Modes in the Histamine H4 Receptor: Linking Ligand-Based Three-Dimensional Quantitative Structure–Activity Relationship (3D-QSAR) Models to in Silico Guided Receptor Mutagenesis Studies, J. Med. Chem., 2011, 54, 8136-8147.
- G. Thoma, M. B. Streiff, J. Kovarik, F. Glickman, T. Wagner, C. Beerli and H. – G. Zerwes, Orally Bioavailable Isothioureas Block Function of the Chemokine Receptor CXCR4 In Vitro and In Vivo, J. Med. Chem., 2008, 51, 7915-7920.
- C. Ma, A. Wu, Y. Wu, X. Ren and M. Cheng, Design and Synthesis of N-Aryl Isothioureas as a Novel Class of Gastric H+/K+-ATPase Inhibitors, Archiv der Pharmazie, 2013, 346, 891-900.
- C. Alonso-Moreno, A. Antinolo, F. Carrillo-Hermosilla and A. Otero, Guanidines: from classical approaches to efficient catalytic syntheses, Chem. Soc. Rev., 2014, 43, 3406-3425.
- T. R. M. Rauws and B. U. W. Maes, Transition metal-catalyzed N-arylations of amidines and guanidines, Chem. Soc. Rev., 2012, 41, 2463-2497.
- P. Mampuys, Y. Zhu, T. Vlaar, E. Ruijter, R. V. A. Orru and B. U. W. Maes, Sustainable Three-Component Synthesis of Isothioureas from Isocyanides, Thiosulfonates, and Amines, Angew. Chem. Int. Ed., 2014, 53, 12849-12854.