Condensation
The pKa of the carboxylic acid is sufficiently high, under non-aqueous conditions, the transfer of the proton between the species is not favored. This is unless the removal of the ammonium carboxylate salt so formed, by its crystallization or precipitation from the solution, drives the proton transfer equilibrium. Where the crystallization or precipitation event does not occur, there is the opportunity for the carboxylic acid and amine to be condensed together through heating. The reaction likely proceeds through the attack of the amine on the hydrogen bond bound dimer form of the carboxylic acid, Scheme 1.The water byproduct from this condensation event needs to be removed to drive the equilibrium to completion and to discourage ammonium carboxylate salt formation. If the solvent and water form a positive, heterogeneous azeotrope, the water can be distilled out using the Dean and Stark method. This is more practical than using a dehydrating agent like a large quantity of molecular sieves.
Scheme 1: Equilibria involved in the thermal condensation of carboxylic acids and amines. The formation of N-benzyl-3-phenylpropanamide is from Williams et al. [2].
Whilst the Dean and Stark approach is atom economical, the elevated temperatures are energy intensive. Additional challenges with this approach are as follows:
- As the condensation is thermally driven, the elevated temperatures used for the reaction may slowly degrade either the coupling partners or the amide product, before the reaction has run to completion. This can include the partial ablation of any stereogenic center next to the carboxylic acid carbonyl.
- The solvents required to force the condensation, which will be immiscible with water, are often not very effective at dissolving up the carboxylic acid and amine coupling partners.
These forcing reaction conditions can be circumvented by activating the carboxylic acid. This installs a leaving group bound to the acyl group of the acid, that can be displaced by the nitrogen of the amine coupling partner, Scheme 2. These protocols differ with respect to their alignment with the twelve principles of green chemistry. This is with respect to both the characteristics of the coupling itself, and the upstream and downstream activities that go into making the reagents or the disposal of the waste products from the coupling.
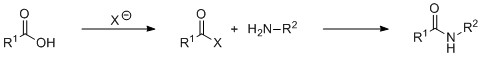
- Charville, D.A. Jackson, G. Hodges, A. Whiting, M.R. Wilson, Uncatalyzed Direct Amide Formation Reaction – Mechanism Studies and the Key Role of Carboxylic Acid H-Bonding, Eur. J. Org. Chem., 2011, 30, 5981-5990.
- L. Allen, A. R. Chhatwal and J.M.J. Williams, Direct amide formation from unactivated carboxylic acids and amines, Chem. Commun., 2012, 48, 666-668.