CHEM21 Case Study: Synthesis of Secondary Amides from N-Substituted Amidines
There is a huge interest in the development of efficient synthesis of secondary amides that involve the construction of C(sp2)-N bond. In the late 1990’s, Ramsden et al. [1][2][3] described a phenyliodine(III) diacetate (PIDA)-mediated oxidative rearrangement of N-substituted amidines, however they found that the reaction product is dictated by the nature of the substituents on the amidine (Scheme 1).[4]
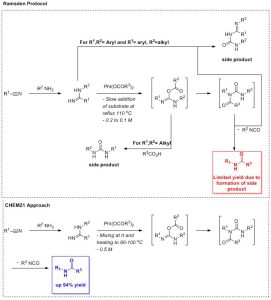
Building on these preliminary results by Ramsden, the CHEM21 researchers investigated whether Ramsden’s protocol could be optimised to allow the efficient, direct and high yielding rearrangement of N‑substituted amidines to their corresponding secondary amides without side product formation. This was achieved by modifying the manner in which the experiment was executed. By using other hypervalent iodine reagents than PIDA, other secondary acetamides were also accessible. N-Substituted amidine substrates are accessible from nitriles through the Pinner reaction or by direct activation with Lewis acids;5 and as such reagents are commercially available, this approach provides a general and simple access route to amides from nitriles and hypervalent iodine reagents.[4]
Through this research, the CHEM21 researchers developed an efficient tandem oxidative rearrangement-elimination reaction that allows access to secondary amines, especially those based on hindered carboxylic acids, or bulky/electron deficient amines that cannot be obtained efficiently from condensation reactions.[4]
- C. A. Ramsden and H. L. Rose, Oxidative rearrangement and cyclisation of N-substituted amidines using iodine(III) reagents and the influence of leaving group on mode of reaction, J. Chem. Soc., Perkin Trans. 1, 1997, 2319-2328.
- M. Bobosikova, W. Clegg, S. J. Coles, M. Dandarova, M. B. Hursthouse, T. Kiss, A. Krutosikova, T. Liptaj, N. ‘a Pronayova and C. A. Ramsden, The oxidative rearrangement of furan-2-carboximidamides: preparation and properties of 2-acylaminofurans, J. Chem. Soc., Perkin Trans. 1, 2001, 680-689.
- C. A. Ramsden and H. L. Rose, Rearrangement and cyclo-[small alpha]-elimination of N-substituted amidines using (diacetoxyiodo)benzene, J. Chem. Soc., Perkin Trans. 1, 1995, 615-617.
- P. Debnath, M. Baeten, N. Lefèvre, S. Van Daele and B. U. W. Maes, Synthesis of Secondary Amides from N-Substituted Amidines by Tandem Oxidative Rearrangement and Isocyanate Elimination, Adv. Synth. Catal., 2015, 357, 197-209.
- T. R. M. Rauws and B. U. W. Maes, Transition metal-catalyzed N-arylations of amidines and guanidines, Chem. Soc. Rev., 2012, 41, 2463-2497.