CHEM21 Case Study: Copper Mediated C-H Activation for the Synthesis of 1,2,3,4-Tetrahydroquinolines
This case study was provided by Ryan Gorman during his time at the University of York.
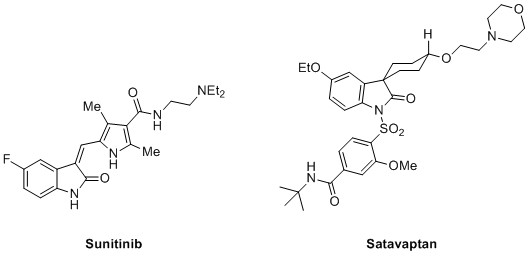
Functionalisation and synthesis of substituted nitrogen containing compounds are one of the top chemical transformations used in industrial processes and medicinal chemistry. [1] In addition to this, is the demand for the development of C-H activation methodologies that negate the need for halogenated starting materials. Nitrogen containing heterocycles represent an important class of biologically active molecules. Fused cyclic systems such as oxindoles are common structural motifs in pharmaceuticals such as the anticancer agent Suntinib and the vasopressin V2 receptor antagonist Satavaptan (Figure 1).[2]
3,4-Dihydro-1H-quinolin-2-one ring systems also display potent biological activity and examples of pharmaceuticals carrying this functionality include the dopamine agonist aripiprazole, the renal deficiency drug trigolutesin A and meloscine used to treat meningitis and heart disease.[2] Added to this, is the extensive bioactivity of 1,2,3,4-tetrahydroquinolines, examples of biologically active molecules include argatroban (potent thrombin inhibitor) and strychnochromine.[2]
The development of synthetic routes to 1,2,3,4-tetrahydroquinolines has seen considerable interest by the synthetic organic community; there have been some recent representative examples for the formation of the C4-C4a bond in particular and these include a Pd0 catalysed cyclopropane C-H activation, [3][4] Povarov reaction, [5][6][7] intramolecular Heck reaction, [8][9] and Ni0 mediated cross coupling [10] among others. Although these methods involve C-H activation of one of the coupling partners, the efficient and direct synthesis of 1,2,3,4-tetrahydroquinolines remains a challenge.
![Scheme 1: Copper mediated C-H activation for the synthesis of 1,2,3,4-tetrahydroquinolines (Taylor et al., 2014[2])](https://learning.acsgcipr.org/wp-content/uploads/2022/07/35c61f6760b21caae66296b6d349005b.jpg)
In an effort to develop such a method in an efficient and sustainable manner, CHEM21 researchers developed a simple copper(II) catalysed method for the synthesis of oxindoles, thio-oxindoles, 3,4-dihydro-1H-quinolin-2-ones and 1,2,3,4-tetrahydroquinolines from linear starting materials by direct C–H, Ar–H coupling (Scheme 1). The method boasts of broad scope in substrate and is carried out in open air using ambient oxygen as the oxidant and thus does not require air and moisture exclusion. The method is shown to be superior to existing methods including protocols mediated by manganese catalysts. [2]
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Analysis of the reactions used for the preparation of drug candidate molecules, Org. Biomol. Chem., 2006, 4, 2337-2347.
- T. E. Hurst, R. M. Gorman, A. Drouhin Pauline and Perry and R. J. K. Taylor, A direct C-H/Ar-H coupling approach to oxindoles, thio-oxindoles, 3,4-dihydro-1 H-quinolin-2-ones, and 1,2,3,4-tetrahydroquinolines, Chemistry, 2014, 20, 14063–14073.
- S. Rousseaux, B. Liegault and K. Fagnou, Palladium(0)-catalyzed cyclopropane C-H bond functionalization: synthesis of quinoline and tetrahydroquinoline derivatives, Chemical Science, 2012, 3, 244-248.
- T. Saget and N. Cramer, Palladium(0)-Catalyzed Enantioselective C-H Arylation of Cyclopropanes: Efficient Access to Functionalized Tetrahydroquinolines, Angew. Chem., 2012, 124, 13014-13017.
- G. Dagousset, J. Zhu and G. Masson, Chiral Phosphoric Acid-Catalyzed Enantioselective Three-Component Povarov Reaction Using Enecarbamates as Dienophiles: Highly Diastereo- and Enantioselective Synthesis of Substituted 4-Aminotetrahydroquinolines, J. Am. Chem. Soc., 2011, 133, 14804-14813.
- A. Mahadev Jadhav, V. Vishnu Pagar and R. – S. Liu, Development of a Povarov Reaction/Carbene Generation Sequence for Alkenyldiazocarbonyl Compounds, Angew. Chem., 2012, 124, 11979-11983.
- P. M. Truong, M. D. Mandler, P. Y. Zavalij and M. P. Doyle, Tetrahydroquinolines and Benzazepines through Catalytic Diastereoselective Formal [4 + 2]-Cycloaddition Reactions between Donor–Acceptor Cyclopropenes and Imines, Org. Lett., 2013, 15, 3278-3281.
- Z. Lu, Y. Cui and Y. Jia, Palladium-Catalyzed Domino Heck/C-H Activation/Intermolecular Direct Arylation Reactions, Synthesis-Stuttgart, 2011, 2595-2599.
- J. E. Wilson, Diastereoselective synthesis of tetrahydroquinolines via a palladium-catalyzed Heck–Suzuki cascade reaction, Tetrahedron Lett., 2012, 53, 2308-2311.
- C. – S. Yan, Y. Peng, X. – B. Xu and Y. – W. Wang, Nickel-Mediated Inter- and Intramolecular Reductive Cross-Coupling of Unactivated Alkyl Bromides and Aryl Iodides at Room Temperature, Chem. Eur. J., 2012, 18, 6039-6048.