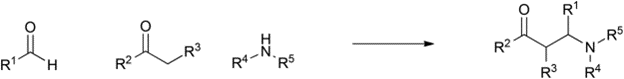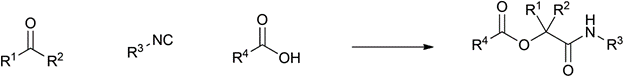Multicomponent Reactions
MCRs (multicomponent reactions) are a valuable strategy in the ‘green chemistry toolbox’ when designing a synthetic approach with sustainability in mind and are increasingly growing in application in medicinal chemistry and drug discovery programmes, combinatorial chemistry, natural product synthesis, and polymer chemistry.[1] They also are ideally suited for diversity oriented synthesis and library generation. In 2014, R. C. Cioc, E. Ruijter & R. V. A. Orru published a review to promote the green process design opportunities available through the application of MCRs [1].
The benefits of MCRs are that they bring together at least three reactants in one-pot bringing about an efficient and intrinsically atom economical reaction (generating a product that contains essentially all the atoms of the starting materials), generally under mild conditions and frequently using greener solvents.[1]
A major advantage of MCRs is waste reduction – due to their highly convergent nature, MCRs reduce waste generated by a process by incorporating a highly resource efficient step in the synthesis and often shortening the overall number of steps. Their excellent chemo-and regio-selectivity minimises the generation of side-products, and by products are typically simple, small molecules such as water, alcohols, amines or common salts, resulting in not only a reduced amount of waste, but the waste itself is generally benign (avoiding problems associated with the recovery and disposal of hazardous waste). The work-up of these reactions is often straightforward via precipitation of the product, avoiding the use of more time consuming and resource intensive recovery and purification methods. Solvent use is also generally significantly reduced due to reaction telescoping and improved work-up.
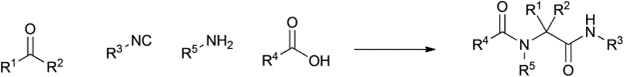
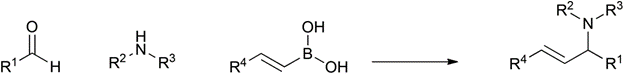
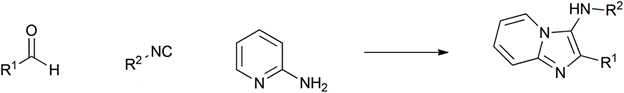
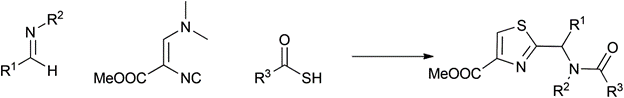
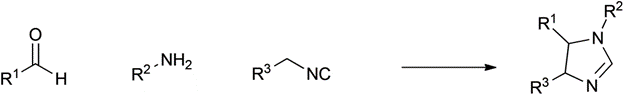
Table 1 demonstrates some representative examples of MCRs and some indicative green chemistry metrics.
Reproduced Content
This material is reproduced from R. C. Cioc, E. Ruijter and R. V. A. Orru, Multicomponent reactions: advanced tools for sustainable organic synthesis, Green Chem., 2014, 16, 2958-2975.
It is copyright to the Royal Society of Chemistry (RSC) and is reproduced here with their express permission. If you wish to reproduce it elsewhere you must obtain similar permission from the RSC.
|
Reaction |
Year |
Scheme |
|
|
Waste |
|---|---|---|---|---|---|
|
Strecker |
1850 |
|
80% |
0.26 |
H2O |
|
Biginelli |
1891 |
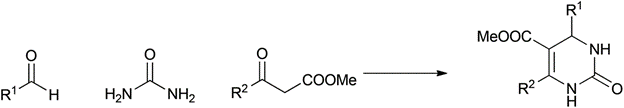 |
84% |
0.20 |
2H2O |
|
Mannich |
1912 |
|
89% |
0.13 |
H2O |
|
Passerini |
1921 |
|
100% |
0.00 |
None |
|
Ugi |
1959 |
|
91% |
0.10 |
H2O |
|
Petasis |
1993 |
|
62% |
0.55 |
B(OH)3 |
|
Groebke-Blackburn-Bienaymé |
1998 |
|
90% |
0.11 |
H 2 O |
|
Passerini-Dömling |
2000 |
|
84% |
0.19 |
Me2NH |
|
Orru |
2003 |
|
86% |
0.16 |
H2O |
One issue with the MCRs is that a number of the MCR strategies use a cyanide or an isocyanide as one of the reaction components, the synthesis of which are typically themselves atom-inefficient and tend to use hazardous reagents and problematic solvents and as such the environmental implications of the preparation of these compounds must be taken into consideration (as well as the toxicity of the cyanide compounds). This reinforces the importance of looking upstream and downstream of a particular reaction step and thinking holistically to ensure that methodologies are genuinely greener. Research is underway to explore more environmentally acceptable methods of generating cyanides, for example the use of potassium hexacyanoferrate (II) as an environmentally benign cyanide source.[2] [3]
- R. C. Cioc, E. Ruijter and R. V. A. Orru, Multicomponent reactions: advanced tools for sustainable organic synthesis, Green Chem., 2014, 16, 2958–2975.
- X. Hu, Y. Ma and Z. Li, Eco-friendly synthesis of α-aminonitriles from ketones in PEG-400 medium using potassium Hexacyanoferrate(II) as cyanide source, Journal of Organometallic Chemistry, 2012, 705, 70-74.
- Z. Li, G. Tian and Y. Ma, One-Pot Three-Component Solvent-Free Cyanoaroylation of Aldehydes Using Potassium Hexacyanoferrate(II) as an Environmentally Benign Cyanide Source, Synlett, 2010, 2010, 2164-2168.