CHEM21 Case Study: Formal Stereoselective C-H Amination
Stereoselective C-H amination is a very attractive strategy for the conversion of simple low cost chemical starting materials to high value chiral amine building blocks, and has therefore attracted much interest in organic chemistry. α-Methylbenzylamine is a substructure of many complex organic molecules and can therefore be a valuable intermediate in their synthesis. [1][2][3]
The most successful chemical strategies reported so far have involved intramolecular and transition-metal catalysed reactions [4] with a number of methods using both chemical [5] and enzymatic [6][7] catalysts. A cascade of two enzymes was reported recently for the terminal amination of fatty acid methyl esters [8] employing a recombinant biosynthetic cascade in a whole cell system. Previous work had already shown that whole-cell biotransformation is able to produce chiral amine from secondary alcohol, albeit in low conversion of 3%.[9]
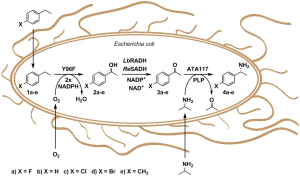
Given that natural biosynthetic cascades often suffer from limited substrate ranges, CHEM21 researchers were interested to generate a de novo biosynthetic multi-enzyme cascade that is guided in design and build by retrosynthetic considerations.[10] The target enzymes for the cascade were carefully chosen to be complementary in substrate recognition. By introducing the pathway into Escherichia coli, CHEM21 researchers generated a biocatalyst which converts ethylbenzenes 1a-e to predominantly (R)-1-phenylethanamines 4a-e respectively (Figure 1) with conversions of up to 26% and ee values of 97.5%. Under the present reaction conditions, no additional co-factor except for the amine donor IPA and molecular oxygen was required. The CHEM21 approach is the first fully enzymatic approach to generate chiral amines at the benzylic position from alkyls.[11]
The flexible generic nature of the design of the cascade will allow for the easy substitution of individual enzymes with homologues or mutants and thereby provide a cassette based modular approach for the design and construction of alternative cascades for the enantioselective C-H amination of other substrates.
- T. C. Nugent and M. El-Shazly, Chiral Amine Synthesis – Recent Developments and Trends for Enamide Reduction, Reductive Amination, and Imine Reduction, Advanced Synthesis & Catalysis, 2010, 352, 753-819.
- A. Henseler, M. Kato, K. Mori and T. Akiyama, Chiral Phosphoric Acid Catalyzed Transfer Hydrogenation: Facile Synthetic Access to Highly Optically Active Trifluoromethylated Amines, Angew Chem Int Edit, 2011, 50, 8180-8183.
- R. I. Storer, D. E. Carrera, Y. Ni and D. W. MacMillan, Enantioselective organocatalytic reductive amination, J Am Chem Soc, 2006, 128, 84-6.
- M. L. Louillat and F. W. Patureau, Oxidative C-H amination reactions, Chem Soc Rev, 2014, 43, 901-10.
- R. A. Green and J. F. Hartwig, Nickel-catalyzed amination of aryl chlorides with ammonia or ammonium salts, Angew Chem Int Ed Engl, 2015, 54, 3768-72.
- C. C. Farwell, R. K. Zhang, J. A. McIntosh, T. K. Hyster and F. H. Arnold, Enantioselective Enzyme-Catalyzed Aziridination Enabled by Active-Site Evolution of a Cytochrome P450, ACS Cent Sci, 2015, 1, 89-93.
- R. Singh, J. N. Kolev, P. A. Sutera and R. Fasan, Enzymatic C(sp)-H Amination: P450-Catalyzed Conversion of Carbonazidates into Oxazolidinones, ACS Catal, 2015, 5, 1685-1691.
- M. Schrewe, N. Ladkau, B. Buhler and A. Schmid, Direct Terminal Alkylamino-Functionalization via Multistep Biocatalysis in One Recombinant Whole-Cell Catalyst, Adv Synth Catal, 2013, 355, 1693-1697.
- S. Klatte and V. F. Wendisch, Redox self-sufficient whole cell biotransformation for amination of alcohols, Bioorg Med Chem, 2014, 22, 5578-85.
- N. J. Turner and E. O’Reilly, Biocatalytic retrosynthesis, Nat Chem Biol, 2013, 9, 285-288.
- P. Both, H. Busch, P. P. Kelly, F. G. Mutti, N. J. Turner and S. L. Flitsch, Whole-Cell Biocatalysts for Stereoselective C-H Amination Reactions, Angew Chem Int Edit, 2016, 55, 1511-1513.
- E. J. Basom, J. W. Spearman and M. C. Thielges, Conformational landscape and the selectivity of cytochrome P450cam, J Phys Chem B, 2015, 119, 6620-7.
- E. O’Reilly, V. Kohler, S. L. Flitsch and N. J. Turner, Cytochromes P450 as useful biocatalysts: addressing the limitations, Chem Commun (Camb), 2011, 47, 2490-501.
- A. Robin, V. Kohler, A. Jones, A. Ali, P. P. Kelly, E. O’Reilly, N. J. Turner and S. L. Flitsch, Chimeric self-sufficient P450cam-RhFRed biocatalysts with broad substrate scope, Beilstein J Org Chem, 2011, 7, 1494-8.
- P. P. Kelly, A. Eichler, S. Herter, D. C. Kranz, N. J. Turner and S. L. Flitsch, Active site diversification of P450cam with indole generates catalysts for benzylic oxidation reactions, Beilstein J. Org. Chem., 2015, 11, 1713–1720.
- V. Erdmann, U. Mackfeld, D. Rother and A. Jakoblinnert, Enantioselective, continuous (R)- and (S)-2-butanol synthesis: achieving high space-time yields with recombinant E. coli cells in a micro-aqueous, solvent-free reaction system, J Biotechnol, 2014, 191, 106-12.
- B. Li, Y. Li, D. Bai, X. Zhang, H. Yang, J. Wang, G. Liu, J. Yue, Y. Ling, D. Zhou and H. Chen, Whole-cell biotransformation systems for reduction of prochiral carbonyl compounds to chiral alcohol in Escherichia coli, Sci Rep, 2014, 4, 6750.
- F. G. Mutti, T. Knaus, N. S. Scrutton, M. Breuer and N. J. Turner, Conversion of alcohols to enantiopure amines through dual-enzyme hydrogen-borrowing cascades, Science, 2015, 349, 1525-9.
- K. Niefind, J. Muller, B. Riebel, W. Hummel and D. Schomburg, The crystal structure of R-specific alcohol dehydrogenase from Lactobacillus brevis suggests the structural basis of its metal dependency, J Mol Biol, 2003, 327, 317-328.
- M. Rauter, A. Prokoph, J. Kasprzak, K. Becker, K. Baronian, R. Bode, G. Kunze and H. Vorbrodt, Coexpression of Lactobacillus brevis ADH with GDH or G6PDH in Arxula adeninivorans for the synthesis of 1-(R)-phenylethanol, Appl Microbiol Biotechnol, 2015, 99, 4723-33.
- C. A. Muller, A. Dennig, T. Welters, T. Winkler, A. J. Ruff, W. Hummel, H. Groger and U. Schwaneberg, Whole-cell double oxidation of n-heptane, J Biotechnol, 2014, 191, 196-204.
- K. Abokitse and W. Hummel, Cloning, sequence analysis, and heterologous expression of the gene encoding a (S)-specific alcohol dehydrogenase from Rhodococcus erythropolis DSM 43297, Appl Microbiol Biotechnol, 2003, 62, 380-6.
- C. A. Muller, B. Akkapurathu, T. Winkler, S. Staudt, W. Hummel, H. Groger and U. Schwaneberg, In Vitro Double Oxidation of n-Heptane with Direct Cofactor Regeneration, Adv Synth Catal, 2013, 355, 1787-1798.
- A. P. Green, N. J. Turner and E. O’Reilly, Chiral amine synthesis using omega-transaminases: an amine donor that displaces equilibria and enables high-throughput screening, Angew Chem Int Ed Engl, 2014, 53, 10714-7.
- E. O’Reilly, C. Iglesias, D. Ghislieri, J. Hopwood, J. L. Galman, R. C. Lloyd and N. J. Turner, A regio- and stereoselective omega-transaminase/monoamine oxidase cascade for the synthesis of chiral 2,5-disubstituted pyrrolidines, Angew Chem Int Ed Engl, 2014, 53, 2447-50.
- M. D. Truppo and N. J. Turner, Micro-scale process development of transaminase catalysed reactions, Org Biomol Chem, 2010, 8, 1280-3.