CHEM21 Case Study: Asymmetric Dihydroxylation
The stereo- and regioselective oxidative functionalisation of olefins is amongst the most challenging reactions in organic chemistry and much effort has been made to develop selective methodologies that cover a broad range of substrates. The asymmetric dihydroxylation is ideally suited for the preparation of chiral building blocks for asymmetric synthesis including the synthesis of pharmaceuticals, fine chemicals, agrochemicals, polymers and natural products.
The most widely applied technique for the preparation of enantiomerically pure cis-diols is the Sharpless dihydroxylation. Using catalytic amounts of osmium(VIII)-oxide in combination with a secondary oxidant and a chiral cinchona alkaloid ligand, various functionalised and non-functionalised olefins of different classes can be converted into their corresponding diols yielding good to excellent stereoselectivities for five of the six alkenes (mono-, gem-di-, trans-di-, tri- and tetra-substituted alkenes). However, in addition to their toxicity, these metal catalysts can also lead to by-product formation due to over-oxidation and cleavage of the diol products.[1][2]
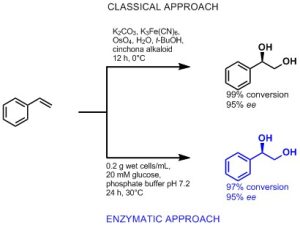
Analogously to chemical strategies, nature has evolved biocatalysts that are highly selective and have been shown to catalyse a wide variety of challenging oxidation reactions. Nevertheless, the diversity of accessible cis-diols is limited by enzyme availability. Despite the vast number of characterised hydroxylating enzymes, only Rieske non-heme iron oxygenases (ROs) display the remarkable ability to stereoselectively introduce two hydroxyl groups in one enzymatic step.[3] Like the well-known P450 monooxygenases, these versatile biocatalysts display a relaxed substrate specificity and furthermore can catalyse various other oxidation reactions including monohydroxylations, dealkylations, desaturations, epoxidations and oxidative cyclizations. [3][4][5][6][7][8]
Rieske non-heme iron oxygenases (ROs) represent promising biocatalysts for oxyfunctionalization as they can be engineered to efficiently catalyse the selective mono- and dihydroxylation of various olefins. The introduction of a single point mutation improved selectivities (≥ 95%) and conversions (> 99%) towards selected alkenes. By modifying the size of the amino acid side chain, CHEM21 researchers were able to modulate the regio- and stereoselectivity of these enzymes. For distinct substrates, mutants displayed altered regioselectivities or even favoured opposite enantiomers compared to the wild type ROs, offering a sustainable approach for the oxyfunctionalisation of a wide variety of structurally different olefins.[8]
- H. C. Kolb, M. S. VanNieuwenhze and B. K. Sharpless, Catalytic Asymmetric Dihydroxylation, Chemical Reviews, 1994, 94, 2483-2547.
- B. Plietker and M. Niggemann, An Improved Protocol for the RuO4-Catalyzed Dihydroxylation of Olefins, Org. Lett., 2003, 5, 3353-3356.
- P. E. Rebecca and R. M. Sol, Applications of Aromatic Hydrocarbon Dioxygenases, in Biocatalysis in the Pharmaceutical and Biotechnology Industries, CRC Press, 2006.
- D. R. Boyd and G. N. Sheldrake, The dioxygenase-catalysed formation of vicinal cis-diols, Natural Product Reports, 1998, 15, 309-324.
- P. K. Sydor, S. M. Barry, O. M. Odulate, F. Barona-Gomez, S. W. Haynes, C. Corre, L. Song and G. L. Challis, Regio- and stereodivergent antibiotic oxidative carbocyclizations catalysed by Rieske oxygenase-like enzymes, Nat. Chem., 2011, 3, 388-392.
- J. Han, S. – Y. Kim, J. Jung, Y. Lim, J. – H. Ahn, S. – I. Kim and H. – G. Hur, Epoxide formation on the aromatic B ring of flavanone by biphenyl dioxygenase of Pseudomonas pseudoalcaligenes KF707, Appl. Environ. Microbiol., 2005, 71, 5354-5361.
- P. Kumar, R. Kumar Upadhyay and R. Kumar Pandey, Asymmetric dihydroxylation route to (R)-isoprenaline, (R)-norfluoxetine and (R)-fluoxetine, Tetrahedron: Asymmetry, 2004, 15, 3955-3959.
- C. Gally, B. M. Nestl and B. Hauer, Engineering Rieske Non-Heme Iron Oxygenases for the Asymmetric Dihydroxylation of Alkenes, Angew. Chem., Int. Ed., 2015, 54, 12952-12956.