C-F Bond Formation
Organofluorine chemistry has a wide variety of applications, from the manufacture of pharmaceuticals and agrochemicals through to polymers and fuel cells. The presence of fluorine atom(s) in an API can provide beneficial effects through increased efficacy, due to for example improved metabolic stability; lipophilicity and/or improved bioavailability through changes to its pKa.[1] Although fluorination remains a critical technology for the pharmaceutical industry, very little is done in house – at least in process and manufacturing.[2]
In 2015 members of the CHEM21 project, Harsanyi and Sandford, based at Durham University published a perspective for the Green Chemistry Journal on fluorine sources, applications and sustainability.[3]
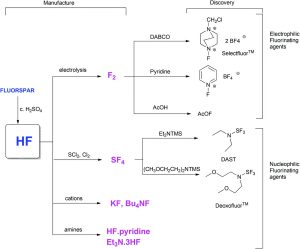
Organofluorine compounds are extremely rare in nature, and therefore the construction of carbon-fluorine bonds requires the use of synthetic fluorinating agents. The range of fluorinating agents used in chemical synthesis (see Figure 1) are ultimately all derived from the mineral fluorospar (CaF2) which is used to make anhydrous hydrogen fluoride (aHF), (see Figure 1).[3]
There is a threat to the long term sustainability of organofluorine chemistry, as estimated reserves of fluorospar are set to last for approximately only another one hundred years. Therefore, alternative sources of fluorine need to be explored. One route that has been suggested is the exploitation of a byproduct of the fertiliser industry ‘fluorosilic acid’, which is formed during the production of phosphoric acid from widely abundant phosphate rock.[3]
- S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Fluorine in medicinal chemistry, Chem. Soc. Rev., 2008, 37, 320-330.
- Fluorination key challenge in API synthesis (Last accessed: August 18, 2022).
- A. Harsanyi and G. Sandford, Organofluorine chemistry: applications, sources and sustainability, Green Chem., 2014, 17, 2081–2086.