Amidation
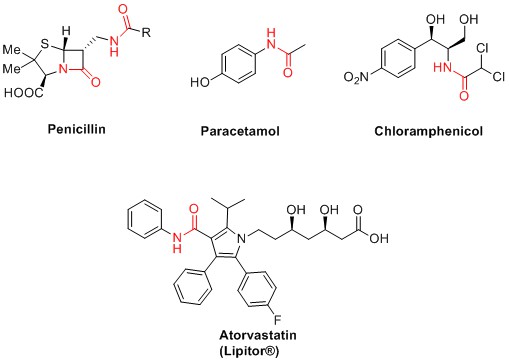
The amide bond is found in natural products, polymers and pharmaceutical molecules. Publications by Carey et al.[1], Roughley et al.[2] and Brown and Bostrőm[3] all include amide bond formation as one of the top transformations in pharmaceutical research and development. Important drug molecules that carry an amide bond include atorvastatin, penicillin, paracetamol and chloramphenicol, Figure 1. The ubiquity of amide bond formation when making pharmaceuticals, animal health products or agrochemicals means its various methods are constantly in the spotlight with respect to their chemical efficiency and green credentials.
- J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Analysis of the reactions used for the preparation of drug candidate molecules, Org. Biomol. Chem., 2006, 4, 2337-2347.
- S. D. Roughley and A. M. Jordan, The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates, J. Med. Chem., 2011, 54, 3451-3479.
- D. G. Brown, J. Bostrőm, Analysis of Past and Present Synthetic Methodologies on Medicinal Chemistry: Where Have All the New Reactions Gone?, J. Med. Chem., 2016, 59, 4443-4458.