Primary Manufacturing
Lifecycle assessment for drug substance
Lifecycle Assessments (LCA) are used by the Sponsor of a new medicine to quantify the environmental impact of its production. Examples of metrics or ‘indicators’ used in these assessments are the total mass of raw materials used to make a kilogram of API, global warming potential (carbon emissions), water depletion (water used), acidification potential (SO2 emissions) and eutrophication potential (nitrites, phosphates), human toxicity potential, ecotoxicity potential and energy consumption. The indicators can be aligned with the Environmental Health and Safety strategy (e.g. reducing energy usage or water depletion) of the Sponsor and its manufacturing partners. The assessment therefore provides a means to identify and thence address parts of the process to be targeted as part of the Environmental Health and Safety strategy.
Learning objectives
- Understand need for clear boundaries to clarify the scope of the exercise
- Understand the four phases of LCA according to ISO 14040
- Build awareness of a streamlined tool for performing the lifecycle assessment of the manufacture of a drug substance.
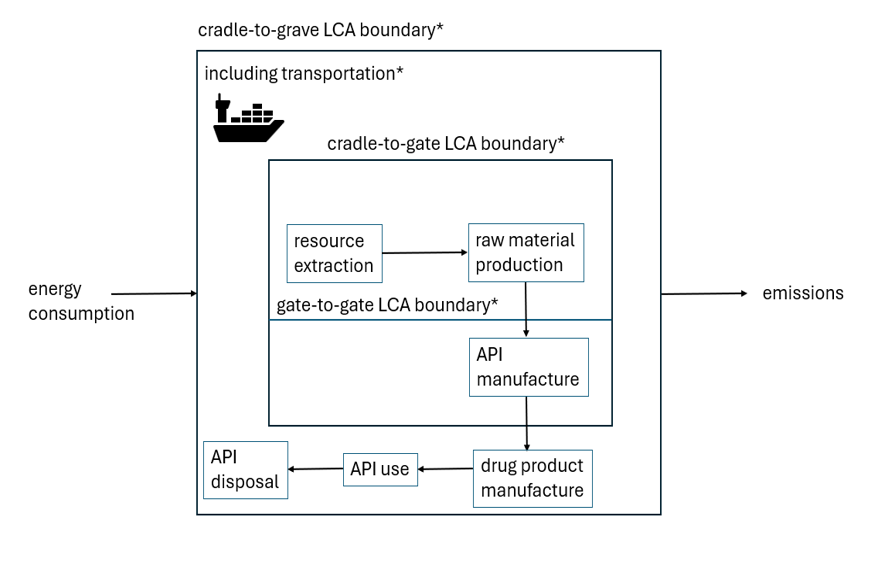
It is important to clarify the scope of an LCA exercise so as to provide context to its recommendations. That is to say, the boundaries of the exercise should be well defined. The various boundaries of a so called ‘cradle-to-grave’, ‘cradle-to-gate’ and ‘gate-to-gate’ exercise are illustrated below. In all of these cases, energy consumption and emissions are out of scope. Operations downstream of the API manufacture are only in scope for the cradle-to-grave analysis.
According to ISO14040, a lifecycle assessment has four stages.
- defining the objectives and scope
- composing a lifecycle inventory using data on material inputs, energy use and emissions
- impact assessment of the lifecycle inventory according to lifecycle indicators
- interpreting the impact assessment according to the goals
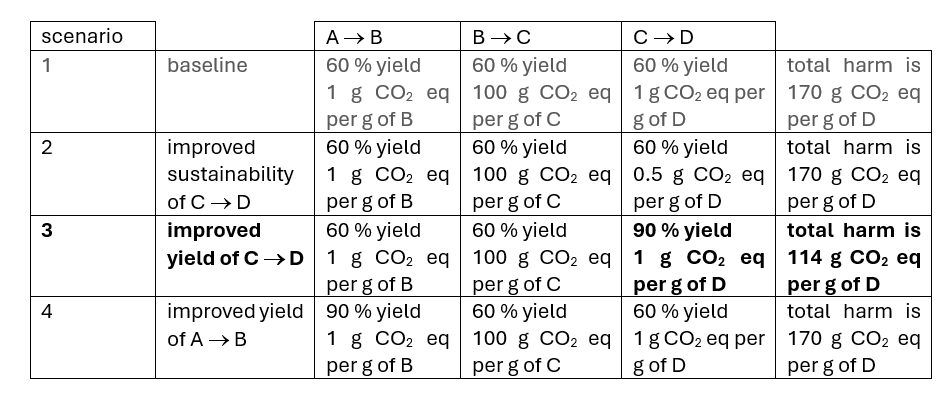
The parts of the process (‘hotspots’) that need to be targeted for improvement may not be the same for each LCA indicator [1]. The assessment should also identify the hotspots in the process that have the most significant impact across all indicators. This often crudely correlates with the contribution to the process mass intensity across the entire product cycle. An indirect hotspot is a transformation in a synthetic sequence downstream of a direct hotspot, and which has a low yield. These characteristics mean that like a direct hotspot, it also has a disproportionate impact on the LCA. This is illustrated in the table below where only scenario 3, an improvement in yield downstream of the direct hotspot (B ® C), offers an overall benefit versus the baseline case of scenario 1
During the Process Design phase of drug substance development, there is not much data available around the process. Only data from laboratory scale runs may be available, and changes to the selection of reagents, solvents and unit operations are being made on a weekly basis.
A full lifecycle assessment is a potentially complex exercise which requires bits of information generated by a number of different supply chain partners. In Process Design, a full LCA does not support timely green-by-design decision-making or the tracking of progress in meeting inhouse process development milestones. Streamlining of the full LCA approach is therefore required [2]. GSK scientists developed the Fast Life Cycle Assessment of Synthetic Chemistry tool to assess the environmental impact of any chemical process based on the quantity and type of materials used [3]. The method ranks processes into three groups (above average, average and below average performance) relative to an internal benchmark of twenty-two optimized processes.
The ACS GCI Pharmaceutical Roundtable have produced their own tool for streamlined ‘cradle to gate’ assessments. It can accommodate a wide variety of linear and convergent processes for the synthesis of small molecule active pharmaceutical ingredients (APIs) from iGAL-aligned raw materials. As part of the streamlining versus a full assessment, material and energy penalties associated with vessel cleaning, waste disposal, packaging or shipping, as well as utility use have been excluded from the tool. Class-average LCI is assigned to reagents depending on its class (organic, inorganic, a transition metal (or lithium) or an enzyme). Ecoinvent data are used for the lifecycle impact assessment of solvents. In the case of the metals, industry-average levels of recovery of the metal are assumed. Conveniently, the methodology uses data that can be acquired in the laboratory, and does not require knowledge of the manufacturing facility earmarked for commercial production.
Case study
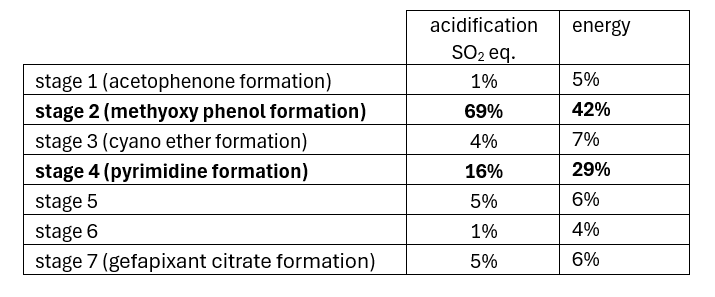
The following discussion relates to the use of the ACS GCI Pharma Roundtable tool to characterize the production of gefapixant citrate (MK-7264) [4]. Details around the first synthesis are captured in red in the scheme. Selected data from the LCA analysis for this first synthesis is tabulated. The indicators used are energy, global warming potential (CO2 equivalents), acidification (SO2 equivalents), eutrophication, water and measure of the mass of resources needed to make the both the API from raw materials and the raw materials themselves
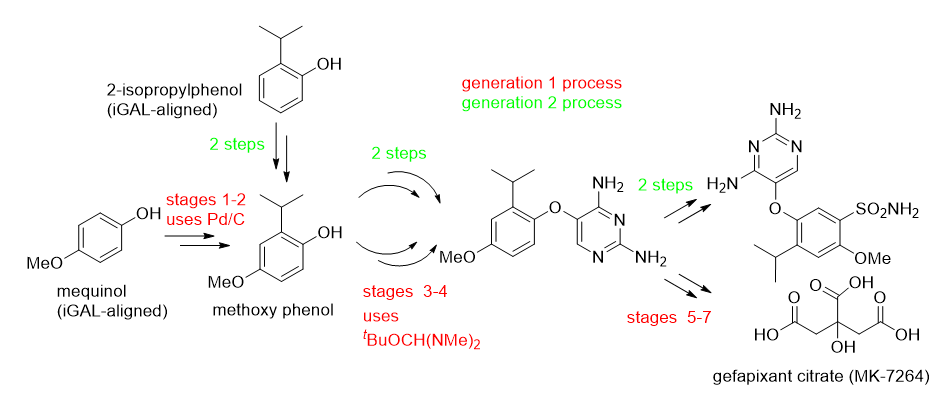
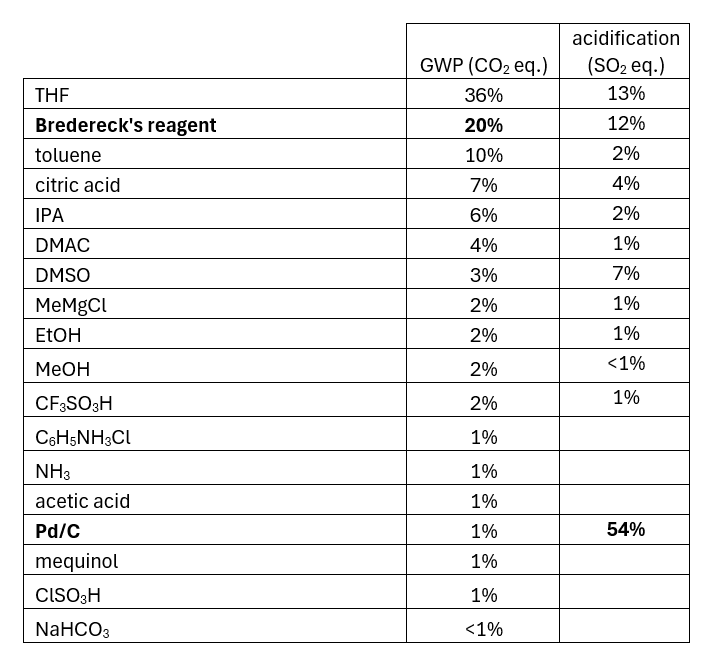
The LCA identified the two most resource-intensive parts of the first synthesis, stages 2 and 4. Focusing on their improvement through the implementation of a second generation approach (summarised in green in the scheme) allowed the PMI for MK-7264 to be lowered from 366 to 88. As part of this, the four transformations that featured in the two stages used to form the methoxyphenol from an iGAL-aligned starting material (mequinol) were reduced to two transformations starting from 2-isopropylphenol. This also allowed the use of Pd on carbon, which dominated the contributions to acidification potential in the original synthesis, to be eliminated. The second generation synthesis also eliminated the use of Bredereck’s reagent from stage 4, which dominated the contribution to global warming potential in the original synthesis.
Lifecycle assessment for drug substance: Summary
- The rigor with which an LCA analysis is performed needs to be phase appropriate. In early development, phase appropriateness can be achieved by making assumptions around the lifecycle inventory data of materials used based on their type.
- As a minimum, the application of an LCA method should allow the identification of “hotspots” that make the biggest contribution to the environmental profile within the boundaries applies to the exercise.
- Fundamental to an organizational culture that supports Green-by-Design Process Design is the provisional of phase appropriate tools that allows the frequent and timely re-evaluation of the LCA of a process. By doing so, areas for improvement are highlighted, guiding the prioritization of process development activities at any stage of the Process Design lifecycle.
- The ACS GCI Pharma Roundtable PMI-LCA tool provides a standard way of performing LCA when a process is in development, but requires knowledge of the entire API synthesis back to iGAL-aligned starting materials.
References
[1] 10.1039/D3GC03601D The need for hotspot-driven research
[2] 10.1039/C6GC02440H Q-SA√ESS: A methodology to help solvent selection for pharmaceutical manufacture at the early process development stage
[3] 10.1065/lca2007.03.315: Fast life cycle assessment of synthetic chemistry (FLASC™) tool
[4] 10.1016/j.crgsc.2022.100324 Green and sustainable metrics: Charting the course for green-by-design small molecule API synthesis
Recommended reading:
D. Ott, D. Kralisch, I. Denčić, V. Hessel, Y. Laribi, P. D. Perrichon, C. Berguerand, L. Kiwi-Minsker and P. Loeb, Life Cycle Analysis within Pharmaceutical Process Optimization and Intensification: Case Study of Active Pharmaceutical Ingredient Production, ChemSusChem, 2014, 7, 3521-3533.
W. De Soete, S. Debaveye, S. De Meester, G. Van der Vorst, W. Aelterman, B. Heirman, P. Cappuyns and J. Dewulf, Environmental Sustainability Assessments of Pharmaceuticals: An Emerging Need for Simplification in Life Cycle Assessments, Environmental Science & Technology, 2014, 48, 12247-12255.
C. Jimenez-Gonzalez and M. R. Overcash, The evolution of life cycle assessment in pharmaceutical and chemical applications – a perspective, Green Chemistry, 2014, 16, 3392-3400.
G. Wernet, S. Conradt, H. Peter Isenring, C. Jimenez-Gonzalez and K. Hungerbühler, Life cycle assessment of fine chemical production: a case study of pharmaceutical synthesis, The International Journal of Life Cycle Assessment, 2010, 15, 294-303.
D. Ott, S. Borukhova and V. Hessel, Life cycle assessment of multi-step rufinamide synthesis – from isolated reactions in batch to continuous microreactor networks, Green Chemistry, 2016, 18, 1096-1116.
- Examining the Life Cycle
- Drivers Towards Whole-process Thinking
- Challenges in Effecting Change
- LCA Examples
- Primary Manufacturing
- Secondary Manufacturing
- Packaging
- Pharmaceuticals in the Environment (PIE)
- Appendix: Carbon Footprinting Assumptions
- Examining the Life Cycle: Quiz
- Examining the Life Cycle: Summary and Further Reading
- The Fate of APIs
- Benign by Design
- Life Cycle Impacts: Summary and Further Reading