Pharmaceuticals in the Environment (PIE)
Over the past 30 years analytical instrumentation and methodology have advanced to a point where we are able to detect very low levels of contaminants in the environment. Increases in detection limits and sensitivity have been driven principally by the development of hyphenated techniques, for example LC-MS-MS and extractive concentration methodology such as solid phase extraction, which has enabled detection and quantitation down to nanograms per litre (ng L-1) levels and lower.
With around 3000 pharmaceutical products in use worldwide (as well as veterinary products and illicit drugs), a large number of pharmaceutical residues have now been detected in a wide range of environmental matrices.[1] [2] [3] These residues, mainly unchanged APIs but also sometimes degradation products, are principally being detected in water and sludge, but have also been found to be present in organisms like fish and plants. [1] [2] [3]
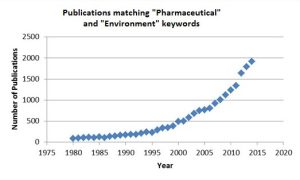
The ability to detect and quantify levels of pharmaceuticals in the environment (PIE) has led to an explosion of interest and scrutiny, as evidenced by the marked increase in the number of publications in this area from 1980 to the present day as shown in Figure 1. Whilst a number of these publications will be on topics like the manufacture and sustainability of pharmaceuticals, the vast majority are focused on the subject of PIE.
PIE have also attracted the attention of a large cross-section of the stakeholders in the pharmaceutical industry ranging from producers, regulators, academics, non-governmental organisations (NGOs), pressure groups and healthcare providers. Whilst bearing in mind objectivity and scientific balance, it is important to be aware of how these issues can be perceived in the public sphere as this can impact upon the reputation of a company, causing knock-on effects.
Recommended reading:
Several examples of PIE appearing in both the scientific literature and public media are given below for further reading.
M. Wu and S. Janssen, Dosed Without Prescription: A Framework for Preventing Pharmaceutical Contamination of Our Nation’s Drinking Water, Environmental Science & Technology, 2011, 45, 366-367.
C. Ort, M. G. Lawrence, J. Rieckermann and A. Joss, Sampling for Pharmaceuticals and Personal Care Products (PPCPs) and Illicit Drugs in Wastewater Systems: Are Your Conclusions Valid? A Critical Review, Environmental Science & Technology, 2010, 44, 6024-6035.
G. M. Bruce, R. C. Pleus and S. A. Snyder, Toxicological Relevance of Pharmaceuticals in Drinking Water, Environmental Science & Technology, 2010, 44, 5619-5626.
How Drug-resistant Pathogens in Water Could Spark Another Pandemic, (Last accessed: September 2022)
There’s Active Drugs in Our Drinking Water: What’s Being Done?, (Last accessed: September 2022)
Pharmaceutical Pollution of the World’s Rivers, (Last accessed: September 2022)
W. Health Organisation, Global Health Risks: Mortality and burden of disease attributable to selected major risks, 2009.
Planet’s Tougher Problems Persist, UN Report Warns (Last accessed: August 2022).
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, M. E. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Pharmaceuticals, Hormones, and Other Organic Wastewater Contaminants in U.S. Streams, 1999−2000: A National Reconnaissance, Environmental Science & Technology, 2002, 36, 1202-1211.
- H. – R. Buser, M. D. Müller and N. Theobald, Occurrence of the Pharmaceutical Drug Clofibric Acid and the Herbicide Mecoprop in Various Swiss Lakes and in the North Sea, Environmental Science & Technology, 1998, 32, 188-192.
- D. Taylor and V. T. Coombe, Environmental and Regulatory Aspects, in Green Chemistry in the Pharmaceutical Industry, Wiley-VCH Verlag GmbH & Co. KGaA, 2010, pp. 83-100.
- Examining the Life Cycle
- Drivers Towards Whole-process Thinking
- Challenges in Effecting Change
- LCA Examples
- Primary Manufacturing
- Secondary Manufacturing
- Packaging
- Pharmaceuticals in the Environment (PIE)
- Appendix: Carbon Footprinting Assumptions
- Examining the Life Cycle: Quiz
- Examining the Life Cycle: Summary and Further Reading
- The Fate of APIs
- Benign by Design
- Life Cycle Impacts: Summary and Further Reading