Scope for Biodegradable API Molecules
One solution put forward to reducing the burden of pharmaceuticals in the environment (PIE) is to design and market totally biodegradable API molecules. Vaccines, therapeutic enzymes, hormones and biological therapies (termed ‘biologics’) like monoclonal antibodies are not subject to ERA, or to as much scrutiny post-patient as small molecule APIs, as they are deemed to be ‘natural’ and rapidly breakdown in the patient and environment to form small non-toxic materials like amino acids.
This may not always be the case with ‘hybrid molecules’ such as PEGylated antibodies and antibody drug conjugates. While the number of biological medicines (biologics) in use has risen over the past 10 years, small molecule APIs are still heavily prevalent for treating many diseases, which suggests that biologics are unlikely to replace small molecules in the near future.[1]
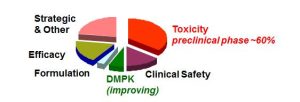
Most pharmaceutical drug candidates fail at the R&D stage (93-96% failure rate), as shown in Figure 1. Early development failure arises from a range of factors such as inadequate pharmacokinetics, bioavailability and unacceptable toxicology profiles, in addition to lack of efficacy in man. Candidate drug properties, a consequence of chemistry design, are key to the success or failure of a proposed molecule. Hence an additional barrier in the form of designing biodegradable drugs would impact further still on what is already a very high attrition rate.
Questions Around Green Design
One solution to potential PIE issues with new pharmaceuticals is ‘green design’; the concept of producing drugs with (near) zero environmental impact, i.e. to exhibit near-zero PBT. This poses a number of questions however:
- Could we implement ‘green design’ without compromising patient benefit?
- Could it apply to all or only some therapies?
- Would we want to ‘design green’? Given the difficulty and large numbers of existing hurdles in getting new pharmaceuticals to market and hence the patient, why would medicinal chemists add further barriers such as designing molecules for degradation post patient?
- When would ‘green benefit’ emerge?
Developing general guidelines is difficult. Lipinski’s famous rule of five describes the desirable ‘drug like’ properties of a molecule:[3]
- No more than 5 hydrogen bond donors;
- Not more than 10 hydrogen bond acceptors;
- A molecular mass less than 500 Da;
- An octanol/water partition coefficient log P of no greater than 5.
At present, a similar guideline for avoiding PBT properties in API molecules does not exist. It is also worth noting that guidelines are not rules – many successful API molecules do not conform to the Lipinski rule of five. Currently, whether or not an API molecule is PBT is generally down to chance, and not a factor of molecular design. A number of strategies to avoid PBT have been put forward for consideration during the molecular design phase, and these will be discussed further in this module.
Example: Glufosfamide
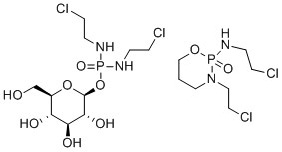
Glufosfamide is often given as an example of a biodegradable version of ifosamide, a drug which is persistent in the environment; the structures of both are given in Figure 2.[4]
Whilst better environmental performance has been suggested in laboratory tests for glufosfamide (which has not so far been marketed), it was not designed to have a greener environmental profile. Ifosfamide has several detrimental issues as an API, including pharmacokinetic variability, resistance and severe host toxicity. Glufosfamide makes use of the normal cell glucose transport mechanism for its own transport into the cell. Glucose transport can be overexpressed and upregulated in certain cancer cell lines, so the ultimate design driver for glufosfamide was to achieve increased selectivity and efficacy in the patient. The better environmental profile was a bonus, as opposed to a design feature, but highlights that the inclusion of natural product-like fragments could result in a better environmental profile.[5]
- Small molecules or biologics? (Last accessed: August 2022).
- I. Kola and J. Landis, Can the pharmaceutical industry reduce attrition rates?, Nat Rev Drug Discov, 2004, 3, 711-716.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings, Advanced Drug Delivery Reviews, 2012, 64, Supplement, 4-17.
- K. Kümmerer, A. Al-Ahmad, B. Bertram and M. Wießler, Biodegradability of antineoplastic compounds in screening tests: influence of glucosidation and of stereochemistry, Chemosphere, 2000, 40, 767-773.
- J. Liang, M. Huang, W. Duan, X. – Q. Yu and S. Zhou, Design of New Oxazaphosphorine Anticancer Drugs, Current Pharmaceutical Design, 2007, 13, 963-978.
- Examining the Life Cycle
- Drivers Towards Whole-process Thinking
- Challenges in Effecting Change
- LCA Examples
- Primary Manufacturing
- Secondary Manufacturing
- Packaging
- Pharmaceuticals in the Environment (PIE)
- Appendix: Carbon Footprinting Assumptions
- Examining the Life Cycle: Quiz
- Examining the Life Cycle: Summary and Further Reading
- The Fate of APIs
- Benign by Design
- Life Cycle Impacts: Summary and Further Reading