Route Selection and Scale Up: Case Study and Exercise
This exercise will provide more information on scale up and why people make particular choices in an industrial setting. It will also explore how we move from a synthetic route that has been developed within medicinal chemistry to a route that is more productive, greener and has a higher throughput. In addition, it will provide an introduction to process safety and the things that you need to look out for when scaling up a chemical route; and good manufacturing practice (GMP). Whilst carrying out this exercise you will need to bear in mind the SELECT criteria.
The Scenario
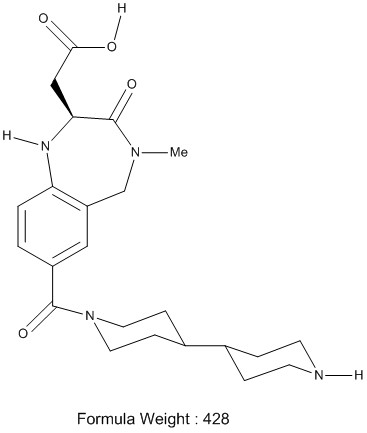
Your company has started a clinical program looking at SB 214857 (Lotrafiban, figure 1) as a prevention for heart attack and stroke. You have scaled up the medicinal chemistry route, with a few modifications, to make multi Kg quantities of drug substance.
Planning for phase III studies, a demand of 3 tonnes (3,000Kg) of API is requested.
Do you stick with the current medicinal chemistry route and scale-up directly, or do you invest time and resource in a new route?
- Route Selection
- GMP
- Introduction to Process Engineering
- Route Selection and Scale Up: Case Study and Exercise
- Process Safety
- Reactive Hazards in Scaling Up: Case Study and Exercise
- Design of Experiments
- Some Definitions
- The Experimental Design Process
- Comparing Traditional Approaches to Experimental Design
- Examples of Variables and Responses for a Chemical Process
- Main Effects and Interactions
- Experimental Designs: Factorial Designs
- Experimental Designs: Response Surface Design
- Design of Experiments: Summary and Further Reading
- Reaction Work-up and Product Isolation
- Environmental Legislation
- Abatement and Waste Treatment