Reductive Amination
The most ideal agent for reductive amination is currently hydrogenation over a supported metal catalyst. Where this can not be used, reagents based on BH3-amine complexes are favoured. Heteroaromatic amine-borane complexes can be generated from the reaction of sodium monohydroxyborohydride, generated in situ (CARE: hydrogen generation), with the parent heteroaromatic amine.
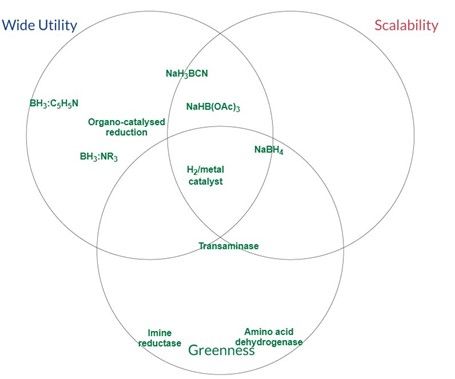
Pyridine borane has a shelf life of only six months and decomposes above 54 °C. Low melting 2-picoline borane (mp 44−45 °C, DSC onset 186 °C) or the liquid 5-ethyl-2-methylpyridine borane (DSC onset 205 °C) are stable replacements and have higher flashpoints than pyridine borane. 2-Picoline borane is available in bulk and is easy to handle on a pilot scale. The stability of both reagents towards hydrolysis or methanolysis allows reactions to be performed in the protic solvents necessary for iminium formation. These reagents also allow the direct charging of a crystalline bisulfite adduct of an aldehyde to the reductive amination. This can be achieved without the need to first reveal the parent aldehyde, a species that is likely to have an inferior stability. A disadvantage of using BH3-amine complexes is that over the course of the reaction and the quench of excess reagent, the offgassing of hydrogen still needs to be accommodated, and there is also the potential for the offgassing of diborane, a highly flammable, reactive and acutely toxic species.
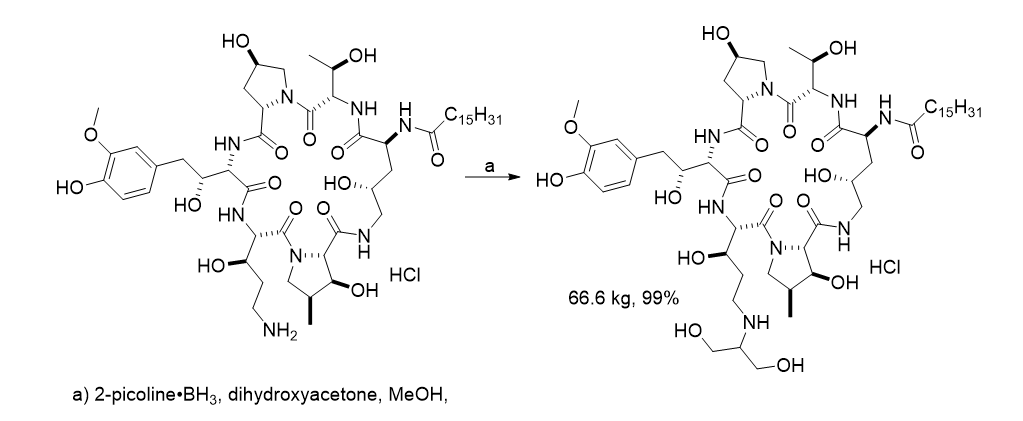
The multikilogram synthesis of ASP4132 used 2-picoline borane [1]. Its use allowed for smooth conversion and the replacement of dichloromethane used in earlier development with THF.
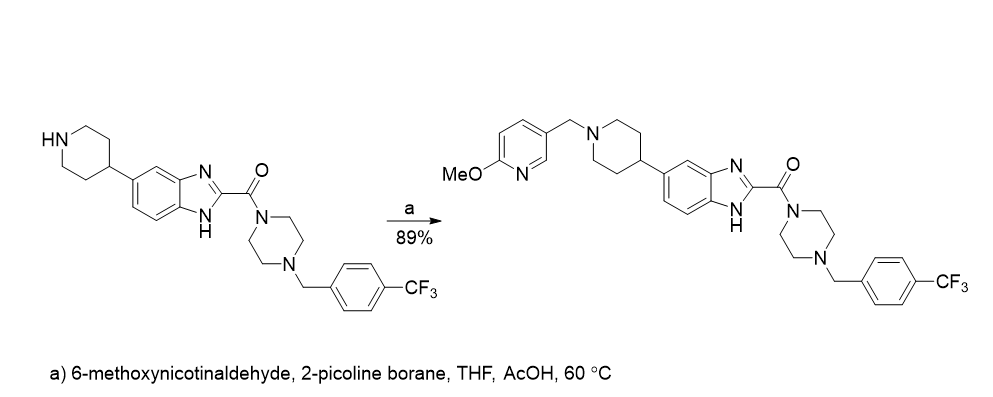
In a second example, 2-picoline borane was used for reproducible reductive amination that featured in the manufacture of the echinocandin ASP9726 [2]. The use of a metal-catalysed hydrogenation proved irreproducible.
Where sodium borohydride can not be used, modified versions of it have disadvantages compared to amine-borane complexes, on top of reacting slowly. Sodium cyanoborohydride poses a toxicity risk, whilst sodium triacetoxyborohydride delivers only one hydride per molecule, possesses low solubility in most organic solvents, and is incompatible with the protic solvents that facilitate imine formation.
In this video, Helen Sneddon describes the application of the GSK reagent guides to a case study on choosing greener reagents for reductive aminations.
[1] Takayoshi Tajima, Hiroaki Hoshii, Yoshinori Kohmura, Development of a Practical and Scalable Synthetic Route for the Adenosine Monophosphate-Activated Protein Kinase Activator, ASP4132, Org. Process Res. Dev. 2021, 25, 2786.
[2] Shinya Yoshida, Joji Hayashida, Yasuhiro Morinaga, Shoji Mizobata, Akihiro Okada, Kazumi Kawai, Shinjirou Tanoue, Tomohide Nakata, Minoru Kitayama, Atsushi Ohigashi, Mitsutaka Matsuura, Takumi Takahashi, Shigeru Ieda, Minoru Okada, A Challenging Synthesis of the Highly Functionalized Echinocandin ASP9726: A Successor of Micafungin, Org. Process Res. Dev. 2014, 18, 725.