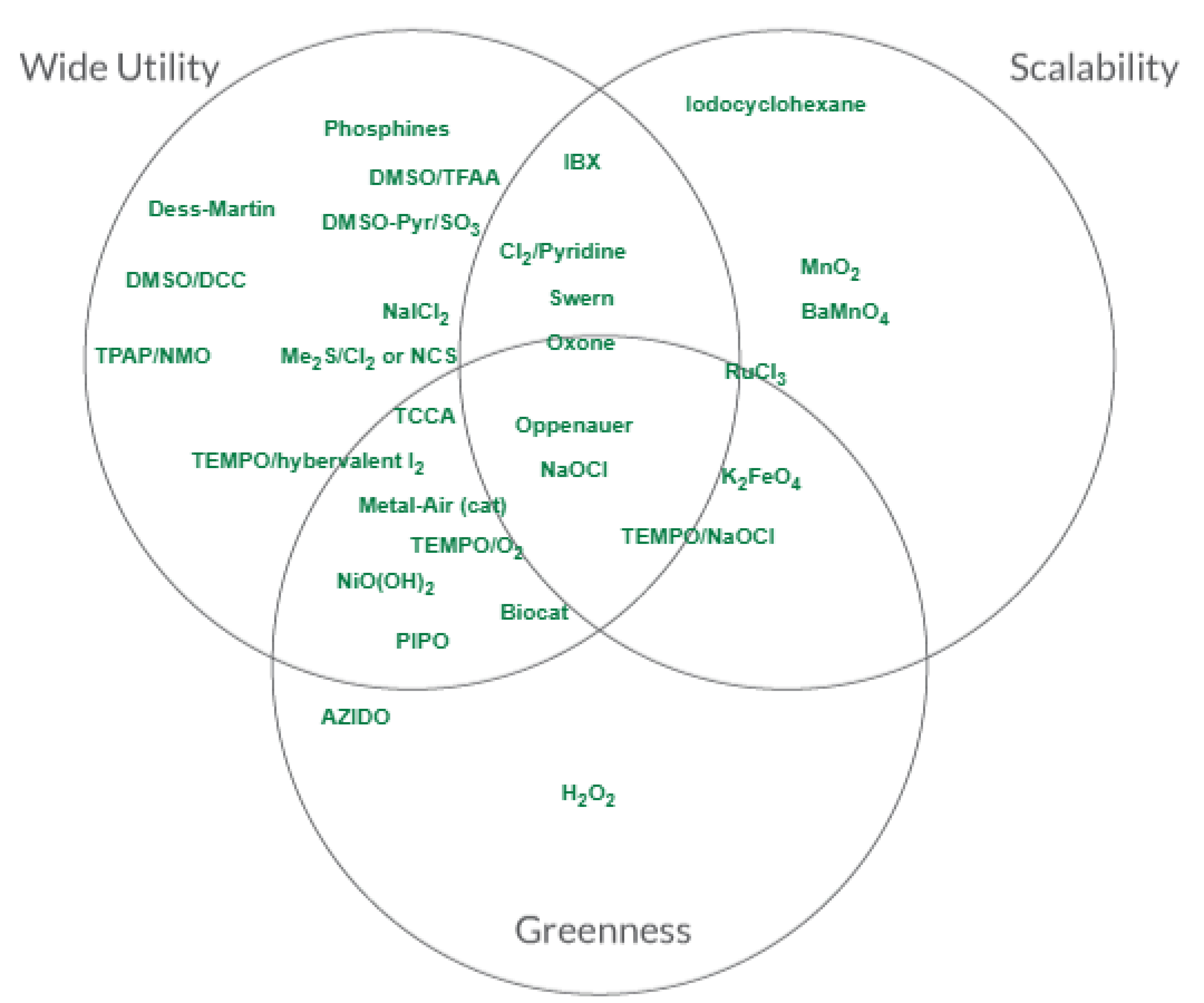
Oxidation to Aldehydes and Ketones
Reagent Guide Example: Oxidation of Alcohols to Aldehydes and Ketones
TEMPO is readily available and has established itself as the catalyst of choice for the oxidation of alcohols in industry [1]. This mild hydroxyl radical catalyst is successful under mild conditions, generally at room temperature. The use of TEMPO can be chemoselective when alcohols other than primary alcohols are present and over-oxidation can be controlled. NaOCl is cheap and is the most utilised co-oxidant for TEMPO in industrial applications, giving rise to safe to safe and scalable conditions when its rate of charging is controlled. Whilst the use of NaOCl occupies the centre of the Venn diagram, examples of its use alone, are rare.[2]
Measures should be put in place to control the presence of metal contaminants, arising either from the surface of a glass-lined reactor or the starting material, as these can reduce the strength of the NaOCl solution. Commercial solutions of NaOCl lose their titre with time and vary in their pH. Using a solution freshly prepared from solid NaOCl·5H2O can allow for the more accurate charging of the reagent and be volumetrically more efficient than using a purchased solution.
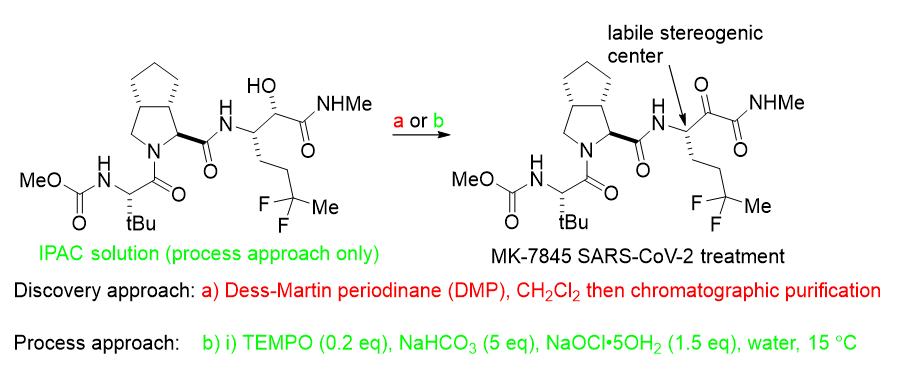
The discovery approach to the SARS-CoV-2 treatment MK-7845 is unsuitable for scaleup as the exotherm associated with oxidation poses risk of violent reaction decomposition due to use of DMP [3]. Also, dichloromethane use is heavily regulated in manufacturing whereas IPAC is a “green” solvent. The Merck process research team sought to replace the use of the DMP reagent with something closer to the center of the alcohol oxidation Venn diagram, the combination of TEMPO and NaOCl. Retaining the IPAC used to extract the starting material in the previous step helped (along with use of NaHCO3) to protect the a-ketoamide stereogenic center from epimerization, and avoided a solvent swap.
In this video, Helen Sneddon describes the application of the GSK reagent guides to a case study on choosing greener reagents for the oxidation of an alcohol to a ketone.
[1] Stéphane Caron, Robert W. Dugger, Sally Gut Ruggeri, John A. Ragan, David H. Brown Ripin, Large-Scale Oxidations in the Pharmaceutical Industry, Chem. Rev. 2006, 106, 294
[2] Stefan Abele, Roman Inauen, Jacques-Alexis Funel, Thomas Weller, Design and Scale-Up of a Practical Enantioselective Route to 5-Phenylbicyclo[2.2.2]oct-5-en-2-one, Org. Process Res. Dev. 2012, 16, 129
[3] Nicholas R. Deprez, Jonathan M. E. Hughes, Shorouk O. Badir, Stasik Popov, Teresa Andreani, Rachel S. Bade, Clara Hartmanshenn, Thomas Tai-min Kwok, Donald R. Gauthier Jr., Nastaran Salehi Marzijarani, Zeinab Sakhaei, Riki Drout, Steve Castro, David J. Schenk, Charles Wolstenholme, Nilusha Padivitage, Cody Welch, Jason R. Kowalski, Brittany Kassim, Yong Liu, Ryan D. Cohen, Alex M. Confer, Guilherme Dal Poggetto, Andrew P. J. Brunskill, Feng Peng, Ji Qi, Jing Xu, Mingxiang Lin, Jamie M. McCabe Dunn, Rapid End-Game Process Development and First GMP Production of MK-7845: An Experimental Antiviral Treatment for COVID-19, Org. Process Res. Dev. 2024, 28, 2157