Process Chemistry versus Medicinal Chemistry
For medicinal chemists, diversity and flexibility of a route is important. They synthesize a wide range of analogues of a lead compound on a small scale (ca. 20mg) for testing in order to increase activity, reduce side effects and to produce an API that may be easily and efficiently administered to the patient. For this, the compound must have the right properties in terms of activity, toxicity, solubility, pharmacokinetics, and pharmacodynamics.
In contrast, process chemistry involves development of practical, safe and cost effective processes for the synthesis of compounds on a larger scale (kg to several tonnes) that have been selected to progress from medicinal chemistry. Therefore, they generally work on a single target molecule and define the best route to that target. Figure 1 below summarizes the key stages in process chemistry for the development of an active pharmaceutical ingredient (API).
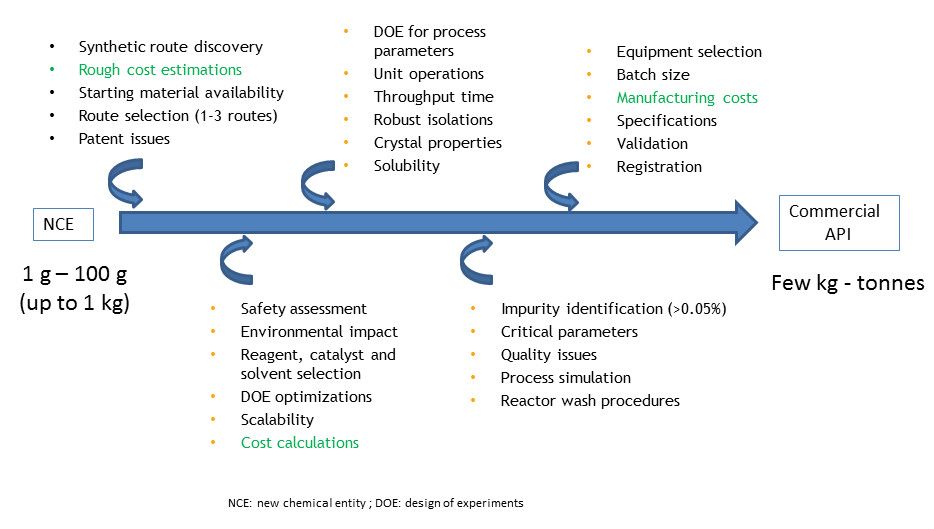
Figure 1: The key stages in process chemistry for the development of an active pharmaceutical ingredient
Case Study
In this video, Esa Kumpulainen describes a literature example of a medicinal chemistry versus a process chemistry route to a target compound.