Case Studies Underpinned by the Considered Use of Solvents
Below are two case studies from the pharmaceutical industry. The desire to eliminate the use of problematic solvents, to cut down on solvent use, either by telescoping steps, performing ‘direct drop’ isolations or choosing solvents with their recovery in mind, enabled highly efficient processes to be employed over the decades since the drugs were launched.
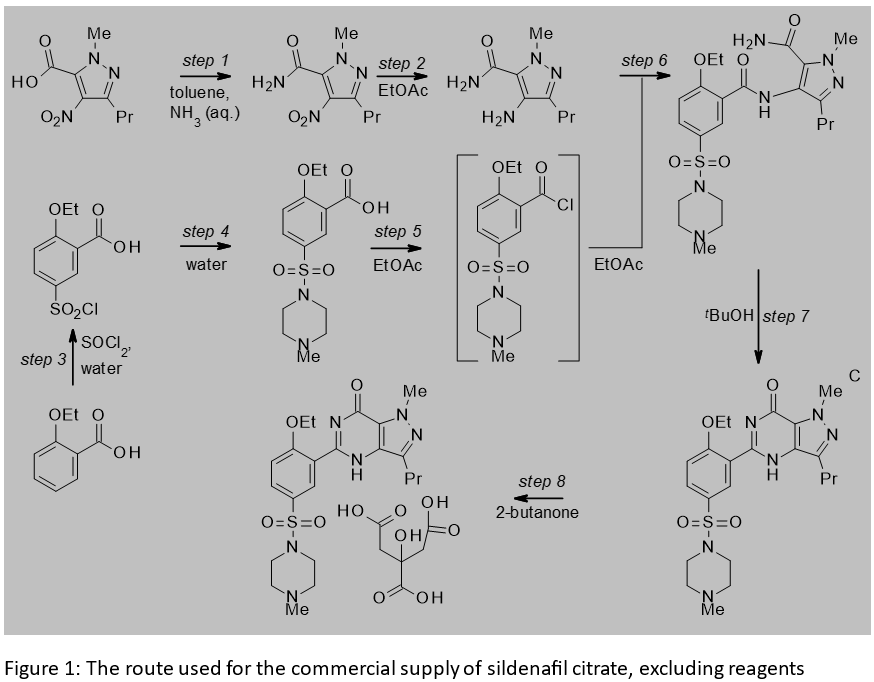
Sildenafil citrate is a drug developed by Pfizer for the treatment of male erectile dysfunction. The convergency of the sequence route used for the commercial supply of sildenafil citrate (Figure 1), its design to incorporate a telescoped sequence and some direct drop isolations combined to slash the amount of waste generated versus the medicinal chemistry route.[1] In step 1 of the commercial route, the excess of thionyl chloride used in the medicinal chemistry route is replaced with a close to stoichiometric amount and the use of toluene to absorb the heat generated. The toluene can be recovered and reused.
2-Ethoxybenzoic acid is a low melting solid and its chlorosulfonylation in step 3 was performed on its melt, avoiding the need to use an organic solvent. At the end of the reaction, treatment with water crystallised the sulfonyl chloride intermediate, ahead of its fresh slurrying in water as part of the sulfonamide formation of step 4.
Steps 2, 5 and 6 constitute a convergent telescoped sequence that is performed in ethyl acetate, which can also be recovered. Despite involving three distinct transformations, the process is simple to operate and the product of the telescope is isolated by a direct drop. The use of this solvent for step 5 could lead to the potential for the inadvertent generation of ethyl chloride with certain reagents used to activate a carboxylic acid. This hazard was eliminated by using carbonyl diimidazole for the activation.
The step 7 process was initially performed in a concentrated solution of tert-butanol. The addition of water as part of the workup made it difficult to recover the tert-butanol. The Pfizer team therefore switched to the use of ethanol, which they found easier to recover than tert-butanol.
In step 8, the crystallization of sildenafil citrate from 2-butanone, a recoverable solvent, replaced the use of water and acetone, a volatile and water-miscible material, used in the medicinal chemistry route. Other highly volatile materials whose use was eliminated across the sequence, versus the medicinal chemistry approach, were diethyl ether, methylene chloride and methanol. When excluding water, but taking organic solvent recycling into account, the amount of organic waste generated per kilogram of sildenafil citrate is only 4 L in the commercial process.
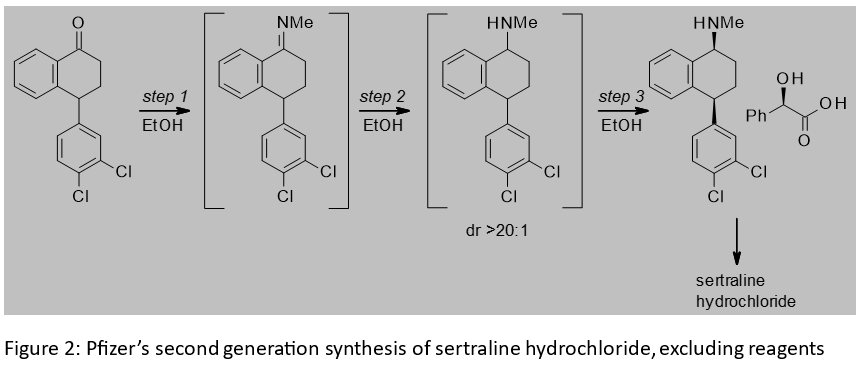
Sertraline hydrochloride is the active ingredient in a Pfizer compound used as an antidepressant. A key intermediate in the synthesis of sertraline hydrochloride is the corresponding mandelate salt. The three step synthesis of this salt from a tetralone starting material is shown in Figure 2. It features an imine formation, imine reduction and diastereomeric salt resolution with mandelic acid.[2] Post-approval improvements to the synthesis resulted in the telescoping of the steps and the use of ethanol throughout the telescope, thus eliminating from the first two steps the legacy use of solvents with an inferior EHS profile to ethanol. Key to the switch to ethanol was the low solubility of the imine in this solvent. Its precipitation drives the imination to completion without the need for an exogeneous dehydrating agent like titanium tetrachloride or molecular sieves. The performance of the imine reduction in ethanol, together with an improved catalyst system, improved the diastereomeric ration versus the first generation route, and reduced byproducts. The product of imine reduction could be telescoped through to the salt resolution with no manipulation other than filtering off the catalyst and decolourizing carbon. The switch to the chemistry in Figure 2 led to a 76% reduction in the volume of solvent used.
- P. J. Dunn, S. Galvin, K. Hettenbach, Green Chem., 2004, 6, 43–48.
- G. P. Taber, D. M. Pfisterer, J. C. Colberg, Org. Process Res. Dev., 2004, 8, 385−38.