Switchable Solvents
So called CO2-switchable solvents make use of aqueous solutions of a water-soluble (poly)amine. In the presence of carbon dioxide, above pressures as low as one atmosphere, the dissolved carbonic acid forms bicarbonate salts [R3NH+][HCO3–], if the (poly)amine is sufficiently basic. This increases the ionic strength, conductivity and the osmotic pressure of the solution. Carbon dioxide is an inexpensive, nontoxic and nonflammable trigger compound. It is also easily removed by heating, sonicating or bubbling an inert gas through the solution, reversing the change in behaviour.
Many solutes are soluble in only one form of the solvent (R3N (aq.) vs [R3NH+][HCO3–] (aq.)), allowing a solute to be dissolved or precipitated by changing the amount of carbon dioxide dissolved in the solvent. Hydrophilic solutes that have previously been dissolved or extracted can later be recovered by expelling them into a separate phase through the addition of carbon dioxide.[1] Alternatively, in the presence or absence of the carbon dioxide, a solute may exhibit a different partition coefficient in biphasic systems formed between an aqueous solution of the ionogen and an immiscible organic solvent. This creates applications for liquid-liquid extraction. The ionic strength of the carbonated aqueous is easily reversed by degassing. This is in contrast to the energy intensive treatment of a saline solution, previously used for “salting out” a solute of interest, prior to discharge to wastewater treatment.
The hydrophobicity and polarity of the amine, the ionic strength and, the concentration of the amine in the water are all levers that can be manipulated in process design. Tertiary amines and their solutions are good candidates as CO2-switchable solvents. The formation of carbamate salts (e.g. [R2NH2+][R2NCO2–] does not compete, as with less substituted amines, and the carbon dioxide absorption is easily reversed which is not the case with more basic compounds like amidines.
An application of a CO2-switchable solvent to the reaction in Figure 1 is now described.[2] The product of the Henry reaction does not partition away from the reaction mixture when washed with water alone. Using a solution of n-butyldimethylamine in carbonated water (amine/water ratio=1:2), the product can be extracted from the postreaction mixture, whilst the catalyst remains in the ethyl acetate reaction solvent. Using the large surface-to-volume ratio of a microextractor to intensify mass transfer, the extraction of product between the two phases takes place with 86% efficiency, ahead of separation of the catalyst bearing ethyl acetate phase in a settler integrated with the setup. Argon gas was used with a falling film microreactor to expel the carbon dioxide from the aqueous phase with a mass loss of 9%, triggering the separating out of the product phase. The product was isolated in 73% yield overall, in 89% ee and with the purity of 98%.
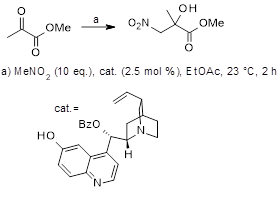
Figure 1: Henry reaction whose workup uses a CO2-switchable solvent
Other applications of CO2-switchable solvents are as follows:
a) Any amidine, a tertiary amine, or bulky secondary amine having a logKow (Kow = octanol–water partition coefficient) between 1.2 and 2.5 forms a biphase if mixed with water.[3][4] If used to dissolve or extract a solute, the amine can be extracted into a carbonic acid solution as [amine•H+][HCO3–], during liquid-liquid extraction. This leaves the solute as an immiscible fraction that can be separated off.[5] Removing the carbon dioxide from the aqueous, causes the “switchable-hydrophilicity” amine solvent to revert to its hydrophobic form which desegregates from water, enabling its recovery and recycle. As the recovery involves liquid-liquid separation, rather than distillation, the amine does not need be volatile, removing flash point and odour risks.
b) The elevated osmotic pressure of the high ionic strength form making it a useful draw solution for the recovery of water from waste water or seawater.
c) In the presence of [amine•H+][HCO3–], the stabilising effect of ionic surfactants, can be turned off, resulting in phase desegregation in a material that was previously an emulsion.
d) There are two component systems like DBU and an alcohol (ROH) that display phase-switchable behaviour in the absence of water. These mixtures can bind carbon dioxide as DBUH+ RCO3–, forcing the desegregating of any other less polar material (decane in Figure 2) that was present alongside the DBU and alcohol, prior to exposure to carbon dioxide.[6]
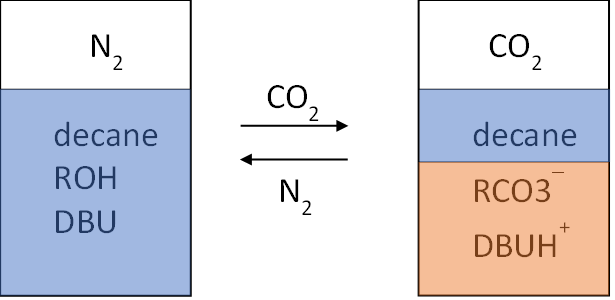
Figure 2: Use of an alcohol and DBU as a CO2-switchable solvent
Recommended reading:
P. G. Jessop, Switchable Solvents as Media for Synthesis and Separations, Aldrichimica Acta, 2015, 48, 18.
- V. S. Liberato, T. F. Ferreira, A. R. MacDonald, B. D. Ribeiro, M. A. Z. Coelho and P. G. Jessop, A CO2-responsive method for separating hydrophilic organic molecules from aqueous solutions: solvent-assisted switchable water, Green Chem., 2023, 25, 4705–4712.
- J. Großeheilmann, J. R. Vanderveen, P. G. Jessop and U. Kragl, Switchable-Hydrophilicity Solvents for Product Isolation and Catalyst Recycling in Organocatalysis, ChemSusChem, 2016, 9, 696–702.
- J. R. Vanderveen, J. Geng, S. Zhanga and P. G. Jessop, Diamines as switchable-hydrophilicity solvents with improved phase behaviour, RSC Adv., 2018, 8, 27318–27325.
- J. Durelle, J. R. Vanderveen, Y. Quan, C. B. Chalifoux, J. E. Kostina and P. G. Jessop, Extending the range of switchable-hydrophilicity solvents, Phys. Chem. Chem. Phys., 2015, 17, 5308–5313
- P. G. Jessop, L. Phan, A. Carrier, S. Robinson, C. J. Dürra and J. R. Harjania, A solvent having switchable hydrophilicity, Green Chem., 2010, 12, 809–814.
- P. G. Jessop, D. J. Heldebrant, X. Li, C. A. Eckert and C. L. Liotta, Reversible nonpolar-to-polar solvent, Nature, 2005, 436, 1102.